Publication in ChemMedChem
Cellular Effects of Cationic Copper(II) Schiff Base Complexes: Anti-Inflammatory and Antiproliferative Properties
Authors: Ján Vančo, Zdeněk Trávníček, Tomáš Malina, Jan Hošek, Zdeněk Dvořák
Full-text: https://doi.org/10.1002/cmdc.202400214
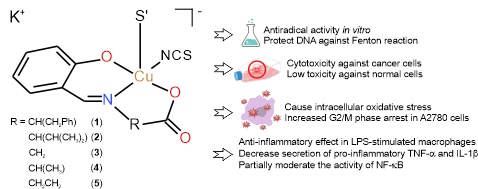
A series of potassium isothiocyanato-(N-salicylidene-aminoaci- dato) cuprates (1–5) with the general formula of the monomeric unit K[Cu(sal-aa)(NCS)]·xH2O (x = 0 or 2), containing a Schiff- base ligand (H2sal-aa) derived from natural amino acids such as glycine, DL-α-alanine, DL-valine, DL-phenylalanine and β-ala- nine, and salicylaldehyde, was screened for in vitro antiradical and major cellular effects against selected cancerous and normal cells. The complexes exhibited strong antioxidant properties against superoxide in vitro and a protective effect on DNA under Fenton-like reaction conditions. Screening of their cellular effects revealed moderate in vitro cytotoxicity against human cancer cell lines (A2780, A2780R and MCF-7), with IC50 values of 25–35 μM, and relatively low toxicity to normal fibroblast MRC-5 cells (with IC50 values> 50 μM). Additional experiments performed on A2780 cells revealed that the most potent complex 5 significantly increased the number of A2780 cells arrested in the G2/M phase of the cell cycle and triggered intracellular oxidative stress. The selected flow cytometry experiments (detection of apoptosis/autophagy and activation of caspases 3/7 and depletion of mitochondrial membrane potential) did not reveal the dominant mechanism underlying the cytotoxicity of the complexes but clearly differentiated their molecular effects from those of the reference drug cisplatin. All the complexes exerted anti-inflammatory effects by modulating the levels of the proinflammatory cytokines TNF-α and IL-1β in LPS-activated THP-1 macrophage-like cells. Complex 5 also slightly influenced the activity of the upstream NF-kB transcription factor, while no effect on PPARγ activation was detected.